Epoxy resins are used in many application, from art to the industrial sector (1).
Epoxies (and its Bisphenol components e.g. BPA) are used in almost every part of our daily life, automotive and marine coatings, water pipes, flooring, paints, electronics, lining tins for tinned foods (2,3), and even some thermal-paper receipts we stick in our pocket when out shopping (4,5,6).
 With all this BPA around, should we be worried?
With all this BPA around, should we be worried?
No need to go shopping in full PPE gear, or wear it when eating a tin of baked beans on the freshly painted bonnet of your car! Just be aware and take care, especially when using the uncured materials. All resins and hardeners show warnings and recommend safety gear when working with the raw materials. More will be explained on Food Safe Epoxy Resin and applications in a future article.
… For Key Points and Quick Read, see the end of the article…
The first epoxies were created by two scientists, Dr Pierre Castan and Dr Sylvan Greenlee in the 1930, used for dental fixtures (1,2) and later became widely used as adhesives. Most epoxies resins are industrially produced petroleum derivatives and most commonly created with Bisphenol A and epichlorohydrin (1). Bisphenol A (BPA) has been around for a long time, first synthesized by a Russian chemist in 1891 (7) and only much later, in the 1950s revolutionized the plastic industry. The BPA (resin) is mixed with a hardener chemical (most often polyamines, aminoamides or phenolic compounds), which reacts and bonds it into a network (the “curing process”) with strong mechanical properties (1). This cured product is often called epoxy resin or just epoxy (although the actual epoxy resin is really the liquid part). And if you want to see how the chemistry works, see the lovely simplified article of “Making Epoxy Resin“.
:: What are the risks?
“Cured epoxy resins do not pose any risk to human health when handled in a professional manner and following the necessary safety measures.” Quote from Epoxy Resin Committee, Europe (1)
The risk of handling epoxies is via skin contact, when mixing and handling the two components, epoxy resin and hardener. Material safety data sheets should always be available and note the warnings on the containers. Epoxies can be handeled safely when taking these precautions (1).
:: Why the fuss about BPA?
Leached Bisphenol A (BPA) was discovered to have weak estrogenic hormone-like properties (3) in the early 1990s (7,5) thus having hormonal active effects when ingested or absorbed. It has been associated with numerous diseases in humans (8) and other living organisms, as discovered since then. Many countries have banned BPA (or associated components) in baby products, especially bottles, as the minute amount of BPA leaching out when a baby bottle or drinking cup is warmed in a microwave, could possibly affect the developing hormonal systems of babies (7,3). Small amounts of BPA leached from food or beverage containers are often ingested by all of us, daily (5).
Of all the BPA produced in the world, more than 99% is converted into polymers, mainly epoxy resins and polycarbons (8). Of this, about 20% is used as a component in epoxy resin (5). “Several high quality migration studies on BPA, which included daily use conditions such as heating, microwaving, machine-dishwashing, rinsing, sterilizing, have repeatedly shown that migration from BPA-based polycarbonate is very low and far below the safety levels set by the authorities. It does not pose a health risk to the consumer under normal handling and use of the products.” Quote from Bisphenol, PC/BPA Group of the industry association PlasticsEurope (8).
The debate on the actual effect of BPA on humans continues. A comprehensive scientific evaluation done by the European Food Safety Authority (EFSA) in 2015 concluded that “scientific knowledge of how BPA behaves in humans was still unclear and there was no single explanation for how BPA potentially affects humans. Therefore, based on the WHO criteria, it was not considered possible to conclude that BPA is an endocrine disruptor”(9). These ideas are constantly challenged. No safety limits have been set for BPA in New Zealand. Safety limits are levels of dietary exposure that are without appreciable risk for a lifetime of exposure (3).
For more on risks, view the Risk Factsheet by the European Food Safety Authority (EFSA) and New Zealand Food Safety Authority’s Bisphenol A – Information Sheet.
:: What about alternatives for BPA?
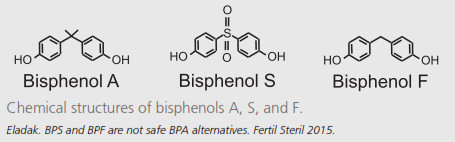
Many alternative chemical compounds have been proposed and is currently in use in epoxy resins, for example* Bisphenol F (BPF) and Bisphenol S (BPS) (5). Both these have been claimed to be safe. Although initially thought to be safe alternatives to BPA, more recent research is showing this not to be the case and the alternatives BPF and BPS can have the same toxic outcomes (5,10). For example, prenatal BPF exposure is associated with decreased cognitive function in children at 7 years, with significant associations in boys (11). BPA substitutes such as BPS and BPF have similiar structures to Bisphenol A and appear to have similiar potencies and actions. BPA substitute-based products (e.g. some epoxy resins used in art) are advertised under the guise of “BPA-free.” This term gives the impression that the products are safe when it is not (12). Most literature has been focussed on BPA and its only now that the new kids on the block, BPF and BPS, are being researched more comprehensively that we are finding them to have the same or similiar toxic effects (10,12).
:: But there is more…
The BPA used in most epoxy resins is a bit different!
BPA is most often combined with another chemical, epichlorhydrin, creating Bisphenol A diglycidyl ether (also known as BADGE) which is a completely different molecule with its own unique properties, and not just a mix of BPA + epichlorohydrin. It is less harmful than pure BPA as shown by the warning icons in the schematic below, and 42 times less soluble in water (2).
:: But my resin SDS does not state BPA or Bisphenol?
Are you sure?
Any chemical substance can have many different synonyms, and the way it is described can be very confusing. Basically, all epoxy resins contain some form of bisphenol base, usually BPA.
Examining the Safety Data Sheets (SDS) of some epoxy resins commonly used in New Zealand resin art, we find they do contain BPA:
| BRAND | COMPONENTS | CAS No. |
WEIGHT % |
| Brand A | 2,2′-[(1-methylethylidene)bis(4,1-phenyleneoxymethylene)]bisoxirane
(which is same as Bisphenol A diglycidyl ether also known as BADGE or DGEBPA) |
1675-54-3 | 60-80% |
| Polyfunctional Epoxy
(same as 1,3-Propanediol, 2-ethyl-2-(hydroxymethyl)-, polymer with 2-(chloromethyl)oxirane) |
30499-70-8 | 5-20% | |
| Bisphenol F
(same as Phenol, polymer with formaldehyde, glycidyl ether) |
28064-14-4 | 1-5% | |
| Brand B | Phenol, 4,4′-(1-methylethylidene)bis-, polymer with 2-(chloromethyl)oxiran
(same as Bisphenol A epoxy resin) |
25068-38-6 | >95% |
| Brand C | Bisphenol-A-(epichlorhydrin)*b | 25068-38-6 | ≥ 80% |
| oxirane, mono[(C12-14-alkyloxy)methyl] derivs. | 68609-97-2 | 10 – 20% | |
| Brand D | Exo-1,7,7-trimethylbicyclo[2.2.1] hept-2-yl acrylate = Isobornyl acrylate |
5888-33-5 | ? |
| Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide = Methanone |
75980-60-8 | ? | |
| 2-((3-((Mercaptoacetyl)oxy)-2,2-bis(((mercaptoacetyl)oxy)methyl)propoxy)methyl)-2-(((mercaptoacetyl)oxy)methyl)-1,3-propanediyl bis(mercaptoacetate) = dipentaerythritol hexakis thioglycolate |
33250-21-4? | ? | |
| Possibly an Acrylic based (BPA free resin) – need more info |
|||
| (a) https://commonchemistry.cas.org for CAS lookups (b) epoxy resins are created by reacting epichlorohydrin (ECH) with bisphenol A, resulting in a different chemical substance known as bisphenol A diglycidyl ether (commonly known as BADGE or DGEBA) |
|||
BPA is such a versatile component, with its incredible properties, which can be manipulated by reacting with other compounds to create a vast array of substances that can be used in industry and products. It is daunting task to find a replacement.
:: So how do we know what’s in my resin?
Some companies make claims of no BPA or any Bisphenol products, so how can we believe them?
- Check the SDS (Material Safety Data Sheet) – and if the supplier doesn’t have it available, request it.
- Next look for the composition. Ignore the fancy chemical formulations, and look up the CAS number.
The CAS Number (Chemical Abstract Service), is a unique numerical identifier assigned to every chemical substance world-wide. A registry of chemicals which shows its structure, its synonyms and more data about the substance. An easy place to check is at Common Chemistry. Sometimes you can even just search the internet for the number and spot all the different names given to the same chemical.
If there is no CAS number on the Safety Data Sheet, be cautious as you have no idea what is in the product!
Key Points:
- Bisphenol A (BPA) or similiar bisphenol components are found in nearly all epoxy resins.
- BPA is used to create epoxy resins and polycarbons; and used very widely in all sectors of industry, plastic bottles, food tins, toothpaste tubes, dentistry, art and more.
- BPA, BPF, BPS and associated bisphenols become inert when mixed with a hardener, but may leach minute amounts that can have possible toxic effect in high concentration. BPF and BPS are not less toxic than BPA.
- Bisphenols are deemed safe by most countries, including USA, Europe, Australia and New Zealand, and does not pose a health risk to the consumer under normal handling and use of the products. Safety procedures required for pre-mixed resin and hardener, as it may cause dermatitis or other skin reactions, or even long term effects. Read the labels.
- The raw liquid Epoxy resins used in art are usually a Bisphenol A (BPA) combined with another compound (e.g. epichlorhydrin) to form a safer (raw) resin compound which then forms a hard network when mixed with the hardener. Once fully cured, there is less chance of BPA leaching out.
- We all consume a very low level of BPA in our lifetime, mainly from migration from BPA-based polycarbonate products; handling thermo-till slips, tinned food, food packaging, pipes and plastics, but far below the safety levels set by the authorities.
- Look up the CAS code to know what is in your epoxy resin.
By Philip Coetzee Ph.D.
May 2024
Future related articles:
→ Epoxy resin and the environment – do you get “green” resin?
→ Food safety, regulations and certification of Epoxy Resin.
References
- Epoxy Resin Committee. (2021, July 17). Epoxies at a Glance. https://epoxy-europe.eu/wp-content/uploads/2015/06/epoxy_resin_committee_factsheet_epoxies_at_a_glance-2.pdf
- Epoxy Resin Committee. (2021, July 17). About Epoxies. https://epoxy-europe.eu/
- New Zealand Food Safety Authority. Ministry for Primary Industries (2021, 18 July). Bisphenol A – Information Sheet. https://www.mpi.govt.nz/dmsdocument/25685-Bisphenol-A-Information-sheet
- Schwarcz, J. (2020, February 25). Turning Up the Heat on Thermal Paper Receipts. https://www.mcgill.ca/oss/article/health/turning-heat-thermal-paper-receipts
- Eladak, S., Grisin, T., Guerquin, Moison, D., Guerquin, M-J., N’Tumba-Byn, T., Pozzi-Gaudin, S., Benachi, A., Livera, G., Rouiller-Fabre, V. and Habert, R. “A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. (2015) Fertility and Sterility, 103 (1), 0015-0282.
- Frankowski, ., Zgoła-Grześkowiak, A. Grześkowiak, T. and Sójka, K., (2020). Environ. Poll. 265, 114879 https://doi.org/10.1016/j.envpol.2020.114879
- Caliendo, H. (2012, June 28). History of BPA. Packaging Digest. https://www.packagingdigest.com/food-safety/history-bpa
- Bisphenol. (2021, 17 July). Science and Risk Assessment. https://bisphenol-a-europe.org/science-risk-assessment/
- Bisphenol. (2017, 13 July). Understanding the debate on endocrine disruptors. https://bisphenol-a-europe.org/understanding-the-debate-on-endocrine-disruptors/
- Usman, A., Ikhlas, S. and Ahmad, M. (2019). Occurrence, toxicity and endocrine disrupting potential of Bisphenol-B and Bisphenol-F: A mini-review. Toxicol Lett. 312, 222-227. https://doi.org/10.1016/j.toxlet.2019.05.018
- Bornehag, C-G., Engdahl, E., Unenge Hallerbäck, M., Wikström, S., Lindh, C, Rüegg, J., Tanner, E. and Gennings, C. (2021). Environ. Int. 150, 106433 https://doi.org/10.1016/j.envint.2021.106433
- Kyong Moon, M. (2019). Concern about the Safety of Bisphenol A Substitutes. Diabetes Metab. J. 43(1) 46-48. https://doi.org/10.4093/dmj.2019.0027
* Other variants include BPG, BPAF, BPE and many more.